Chemical Stability
Assay information
Compound Requirements
25μL 10mM DMSO stock
Time Points
T= 0, 30, 60, 1440 minutes
Assay Media
SGF, pH 1.2
SIF, pH 6.5
1X PBS, pH 7.4
Universal Buffer, pH 9.0
Source Plate Temperature
20°C or 37°C
Quantitation Method
HPLC-UV
Data Delivery
% Remaining at each time point relative to T=0
Identify compound liabilities early by assessing stability in varying environments
Highly unstable compounds can lead to ambiguous in-vitro biological data and may not be suitable candidates for development. Our automated, short-term chemical stability assay mimics the gastrointestinal transit time and in-vitro biological conditions, delivering stability-pH profiles. Use this assay to identify liabilities early, avoid ambiguous data, and develop appropriate compound modification strategies before the compound progresses.
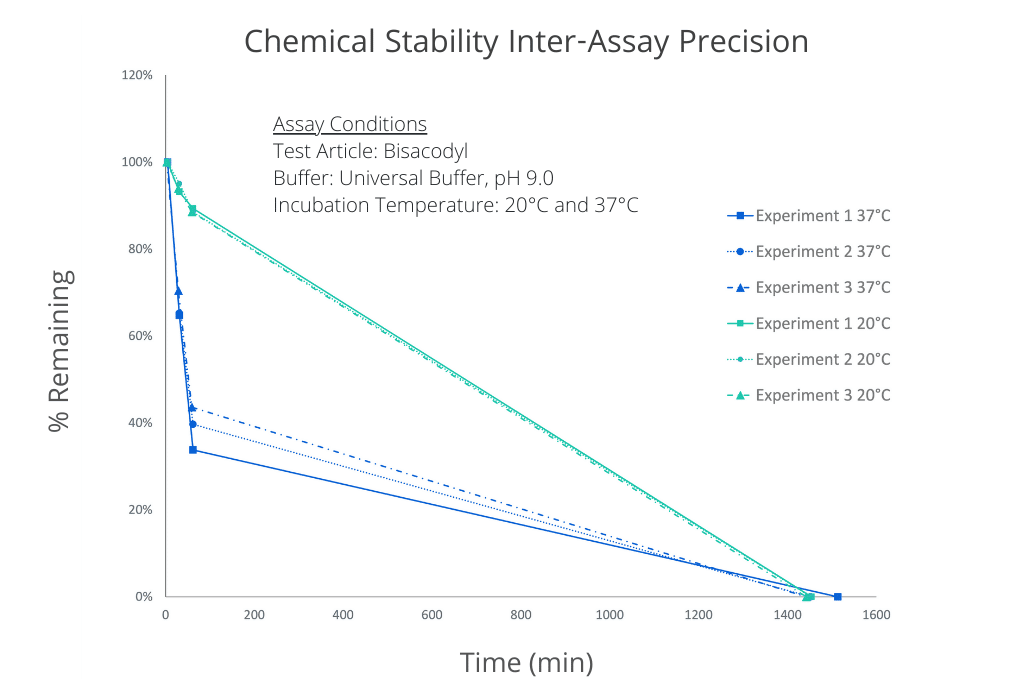
The inter-assay precision of the chemical stability assay is demonstrated below as the overlayed plots of % remaining vs. Time. The assay was performed on Bisacodyl on 3 separate occasions spanning a period of several months. Temperature dependence is also illustrated.
Contact us to learn more about our capabilities or to get a quote for your project.
