Plasma Stability
Assay information
Compound Requirements
10μL 10 mM DMSO stock
Time Points
Screening: 0 and 15, 30, or 60 min.
Half Life: 0, 15, 30, 60, and 120 min.
Species
Rat
Mouse
Dog
Human
Analysis Method
HPLC-MS
Data Delivery
Screening: Percent Remaining
Half Life: Intrinsic Clearance
Poor plasma stability can lead to rapid clearance, short half-lives, and poor in-vivo performance
Poor plasma stability can lead to rapid clearance, short half-lives, and poor in-vivo performance. Pharmacokinetic studies are especially challenging for these compounds as they continue to degrade even after blood is sampled from the study animal, leading to ambiguous data. In the case of prodrugs and antedrugs, rapid degradation in plasma is desirable.
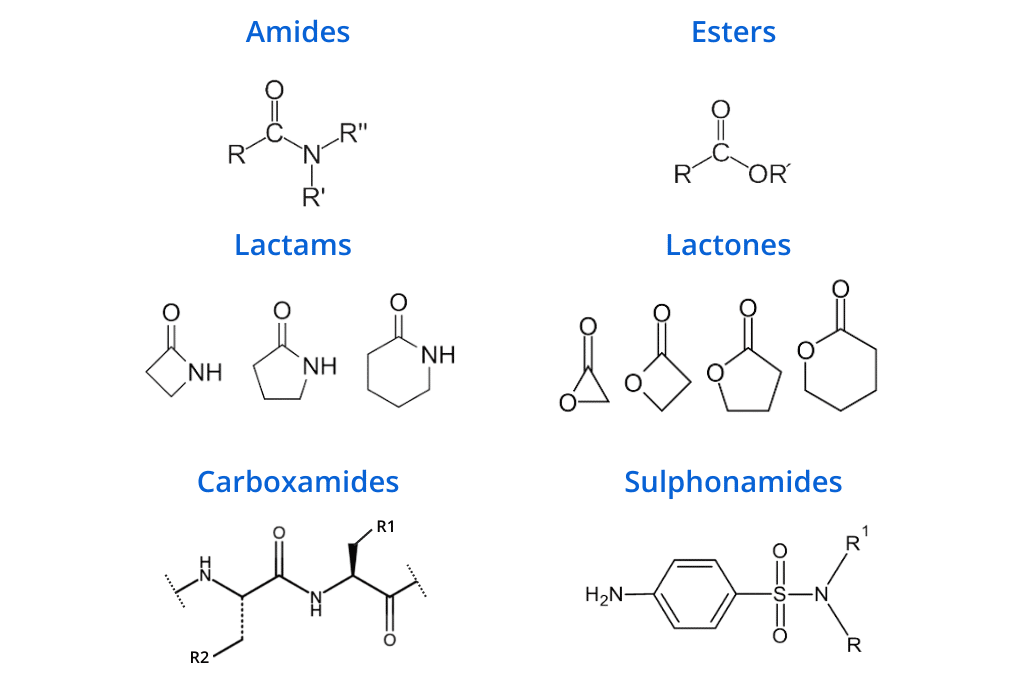
If your compounds contain these plasma-labile functional groups, plasma stability should be studied:
Contact us to learn more about our capabilities or to get a quote for your project.
